A team, led by the London School of Hygiene & Tropical Medicine, has uncovered the strongest evidence yet that septins take Shigella prisoner.
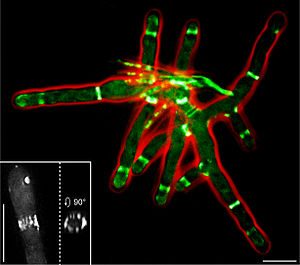 It reveals for the first time that these proteins can detect where bacteria will split for division and prevent it from doing so by forming cage-like structures around the bacteria.
It reveals for the first time that these proteins can detect where bacteria will split for division and prevent it from doing so by forming cage-like structures around the bacteria.
The research team say that although septins are a powerful, natural mechanism to restrict Shigella, future work is required to determine how septin biology can be harnessed for therapeutic purposes. It is hoped that these new findings may lead to a novel way to boost the human immune system and treat a wide variety of bacterial infections.
Lead author Professor Serge Mostowy from the London School of Hygiene & Tropical Medicine said: “We are actively working to engineer this discovery for human health application. If we can use drugs to boost septin caging, we have a new way to stop infection.”
In 2010, researchers first observed that septin cages can entrap Shigella, opening up the tantalising prospect of a new way to stop the bacteria spreading in the body. However, how cells recognise Shigella for entrapment, and the fate of entrapped bacteria, was mostly unknown.
The authors acknowledge limitations of the study including the possibility that some bacteria have evolved to avoid septin cage entrapment, and the need for in vivo study prior to application in humans.
